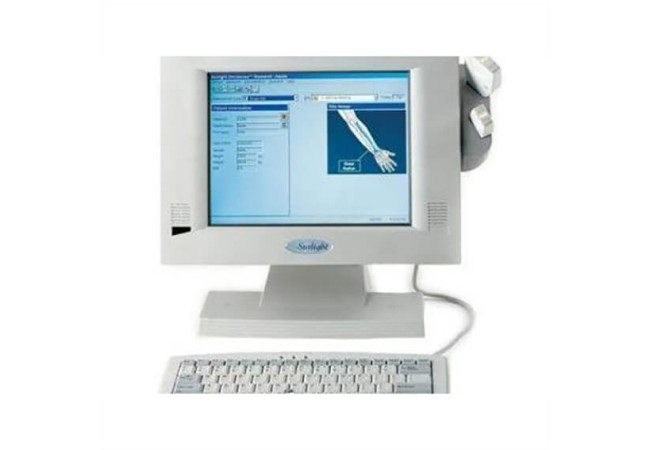
Sunlight Omnisense® 8000S is a high-end mobile bone assessment device that delivers high precision and accuracy along with user-friendly, radiation-free measurement at multiple skeletal sites. Omnisense 8000S offers complete portability, providing the ideal mobile bone assessment solution for the clinics and physicians that require it.
Omnisense 8000S is the only portable bone assessment device on the market today with measurement at multiple skeletal sites. Measurement with Omnisense 8000S is based on Omnipath®, a patented technology developed for diagnosing and monitoring osteoporosis. With its convenience in portability, quick-set-up, and compactness, Omnisense 8000S can also be used as a shared resource or a mobile service.
Omnisense 8000S is a compact self-contained device based on a familiar Windows user interface. The Omnisense concept embodies unique patient history and scheduling features which enable you to easily track measurement history and monitor treatment.
Features and Benefits:
- Compact and portable.
- Measurement at multiple skeletal sites.
- Ethnicity-based male and female reference databases.
- Fits WHO criteria for osteoporosis diagnosis.
- FDA and CE approved.
Materials Included:
- Omnisense 8000 Main Unit with Built-in Monitor.
- Probe
- Spring Gauge
- Phantom for Quality Verification
- Hand Rest
- Skin Marker
- Ultrasonic Gel
- Keyboard
- Mouse
- Power Cord
- Trolley
- Carrying Case
- User Manual Parameter
Measurement Precision and Accuracy : RMS CV = 0.4% - 0.8% in-vivo precision, depending on site, 0.25% to 0.5% instrumental accuracy, depending on probe.
Measurement Sites :
- Distal 1/3 radius (forearm)
- Proximal phalanx III (finger)
- Metatarsal V (foot)
- Mid-shaft tibia (lower leg)
Technology : Quantitative ultrasound, using Omnipath® axial transmission technology.
Measured parameter : Axially transmitted speed of sound (SOS), expressed in m/sec.
Scan time : Less than one minute per skeletal site.
Data Analysis : Compares SOS results with reference database and reports T-scores and Z-scores.
Regulations and Certifications : IEC 60601-1, IEC 60601-1-2 Class B, Part 15 of the FCC rules, Class B, CE Mark.
Display : 12.1" TFT color LCD display.
User Interface : Mini keyboard, integrated mouse.
Power : 100-240V (autoswitchable), • ~50-60 Hz.